Appearance
色谱法(Chromatography)
- 贡献者:郑桂琳;整理:陈宇喧
In this section, two types of chromatography are presented: TLC and flash chromatography.
在本节中,介绍两种色谱类型:薄层色谱和急骤层析。
薄层色谱法 (Thin Layer Chromatography)
参考文献Reference: Wall, P. E. Thin Layer Chromatography; Royal Society of Chemistry: Cambridge (UK), 2005.
The thin layer chromatography (TLC) plates that we buy are slide glasses coated with silica gel. In order to visualize UV-active organic compounds, the silica gel is coated with a layer of fluorescent dye "Flur". Therefore, UV-active compounds can be detected under UV light. Occasionally, non-UV-active compounds are also seen. One case is iodides, which are UV-light quenchers, while another case is inorganic salts, often seen on the baseline; the salts are visualized under UV simply because they cover the fluorescent dye "Flur".
一般来说市售薄层色谱(TLC)板是涂有硅胶的玻片。为使紫外活性的有机化合物可视化,硅胶涂有一层荧光染料“Flur”。因此,在紫外光下可以检测到具有紫外线活性的化合物。人们偶尔也能看到非紫外线活性的化合物:一种情况是碘化物,它是紫外线淬灭剂,另外一种情况是无机盐,通常出现在基线上;这些盐在紫外线下可见是因为它们覆盖住了荧光染料“Flur”。
The TLC plates that we buy are somewhat too large. It is a good idea to cut the plates with a diamond-type or carbide roller into smaller plates. Not only does this save costs, it is also faster to develop a smaller TLC plate than a larger one.
有些时候买到的TLC板子有点大,这时可以用金刚石型或硬质合金辊将TLC板切成较小的板子。这不仅可以节省成本,而且开发小型的TLC板,跑起来也比大的TLC板子快一点。
Retention factor, Rf, is a measurement of how far up a plate the compound travels. Rf is calculated by the distance of the center of the spot from the baseline (P or SM) divided by the distance of the solvent front from the baseline (S). Column volume (CV) is 1/Rf (Figure 1.3). These values will be referenced in the discussion of column chromatography.
保留因子(比移值[ 药典里,TLC法的Rf称为“比移值”。])Rf,可用于表示化合物在平板上移动的情况。Rf计算公式为基线至展开斑点中心的距离(P或SM)除以基线至展开剂前沿的距离(S)。柱容积(CV)为1/Rf(图1.3)。这些值将会被用于讨论柱层析的情况。
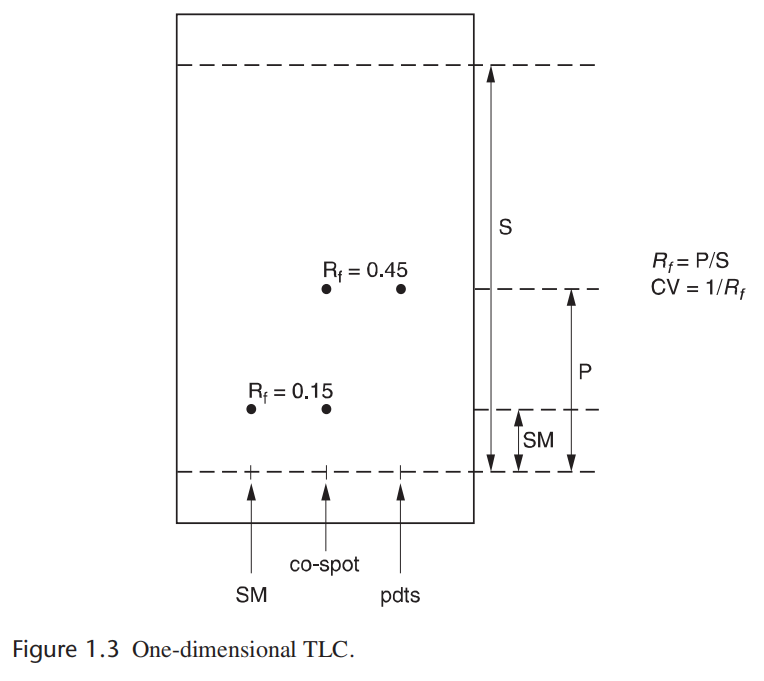
图1.3 一维薄层色谱
In general, the eluting strength of commonly used solvents for normal phase chromatography is: stationary phase = silica gel neutral alumina; increasing order proceeds as follows: petroleum ethers < hexanes < cyclohexane < toluene < diethyl ether < dichloromethane < chloroform < ethyl acetate < acetone < ethanol < methanol < acetic acid.
一般情况下,常规相色谱常用溶剂的洗脱强度为:固定相=硅胶中性氧化铝;展开剂依次增强顺序为:石油醚 < 己烷 < 环己烷 < 甲苯 < 二乙醚 < 二氯甲烷 < 氯仿 < 乙酸乙酯 < 丙酮 < 乙醇 < 甲醇 < 乙酸。
Another useful technique is two-dimensional TLC (Figure 1.4). This method is a simple way of determining if decomposition of a desired product or products will occur on the stationary phase (typically silica gel or alumina). After spotting your compound on the TLC plate and developing the plate, the plate is turned 90º and then developed again. For a single component, one spot should be visible with the same Rf as the spot from the first development. If streaking is observed in the second development and/or new spots arise, decomposition has occurred. For compound mixtures, the final developed spots will fall in a straight line if no decomposition has occurred; however, if the spots do not fall in a straight line or additional spots appear within a lane, decomposition occurred during TLC development. Decomposition is usually an indication that a chemical interaction occurred with the stationary phase.
另一个有用的技术是二维TLC(图1.4)。这是一种确定所需的产物是否会分解或产物是否会在固定相(通常是硅胶或氧化铝)上发生分解的简单方式。在薄层色谱板上点样并经展开、显影,将板转90度,然后再次展开、显影。对于单个组分,一个斑点应该与第一次显影时的斑点具有相同的Rf;如果在第二次展开中观察到条纹和/或出现新的斑点,则已经发生分解。对于复合混合物,如果未发生分解,最终显影斑点将呈直线分布;但是,如果斑点不在一条直线上,或者在一条线上显现出额外的斑点,则说明TLC展开过程中发生了分解。如遇分解,则通常表明与固定相发生了化学相互作用。
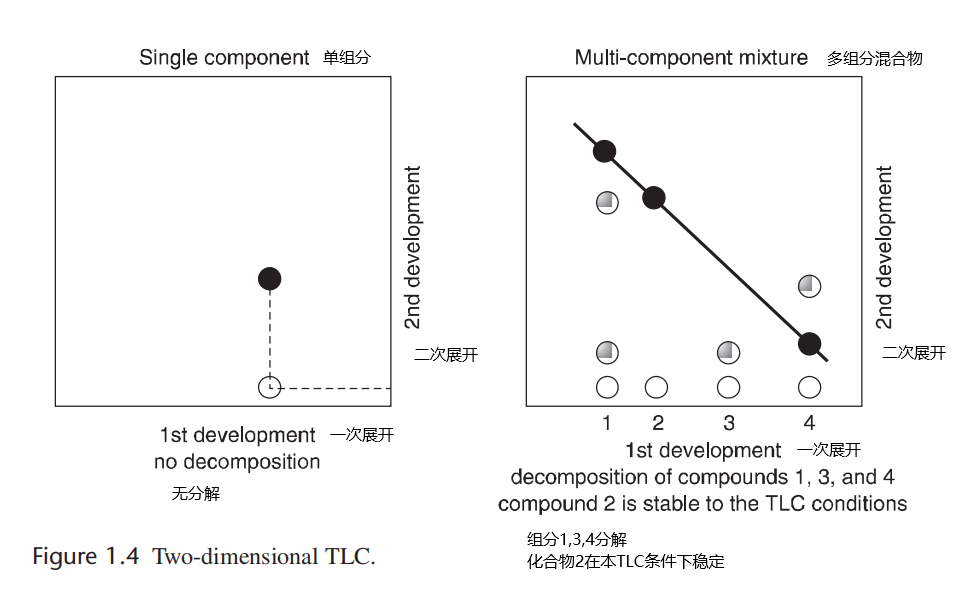
图1.4 二维薄层色谱
普通薄层色谱染色的配方 Recipes of Common Thin Layer Chromatography Stains
For compounds that do not visualize well under UV, TLC stains are necessary to visualize the spots. Listed herein are some popular TLC stains often used in an organic chemistry laboratory. Most of these visualization stains require the submersion of the TLC plate in a solution of stain, carefully blotting excess stain, and then heating the stained TLC plate on a hot plate or with a heat gun. All of these manipulations should be performed in a well ventilated hood, particularly the heating step, because many of these stains are toxic!
对于在紫外线下不能很好地观察到的化合物,薄层色谱染色是使化合物可视化的重要手段。这里列出的是一些薄层色谱染色剂,常用于现代有机化学试验室。大多数可视化染色需要将TLC板浸入在染色溶液中,仔细吸收过量的染色剂,然后在热板或用热枪加热染色后的薄层色谱板。所有的加热操作应该在通风良好的通风罩中进行,特别是加热步骤,因为很多这些染色剂都是有毒的!
MODERN ORGANIC SYNTHESIS IN THE LABORATORY
有机合成技术是在实验室里进行的
对甲氧基苯甲醛染色剂 p-Anisaldehyde Stain
The greatest advantage of this stain is that different colors are manifested on TLC on heating for different molecules. Therefore, molecules can be differentiated even if they have the same Rf values. The disadvantage is its strong but pleasant odor release during heating (toxic, in the hood!).
这种染色剂最大的优点是加热条件下薄层色谱可以将不同的化合物显示出不同的颜色。因此,即使它们有相同的Rf值,分子也可以分化。其缺点是它能释放出刺激但令人愉快的气味加热期间(有毒,在发动机罩内!)。
p-Anisaldehyde(对甲氧基苯甲醛) 12 g (11 mL)
Ethanol(乙醇) 500 mL
Concentrated H2SO4(浓硫酸) 5 mL
硫酸铈染色剂(Cerium Sulfate Stain )
General stain. Most compounds are stained brown or yellow. Cerium Sulfate (8 g)
15% sulfuric acid (100 mL)
常规的染色剂。大多数化合物被染成棕色或黄色。
硫酸铈 (8 g)
15%硫酸 (100 mL)
铈-钼酸铵染色剂(钼酸铵染色剂)- Hanessian Stain (cerium molybdate stain)
One of the most sensitive stains which detects most functional groups. The disadvantage is that everything stains blue.
最能敏感检测到大多数官能团的的染色剂之一。缺点是所有化合物都被染成蓝色。
Ce(SO4)2(硫酸铈) 5 g
(NH4)·6Mo7O24·4H2O(七钼酸铵) 25 g
Concentrated H2SO4(浓硫酸) 50 mL
H2O 450 mL
碘或碘和硅胶 - I2 or I2 in Silica Gel
Everything stains yellow. Solid I2 can be added to a developing chamber and the TLC plate developed by placing the plate in the chamber. Alternatively, the I2 can be dispersed on silica gel in a developing chamber, and the TLC plate immersed in the silica gel; the silica gel evelopment tends to be faster. The spots will fade, but the plate can be redeveloped by placing the plate back into the I2 chamber.
该染色剂将有机物都染成黄色的。可以将薄层色谱板放入加入固体碘的显影室中。或者,显影室里的碘也可以分散在硅胶上,而薄层色谱板浸在硅胶中;硅胶会使爬板更快。这些斑点会褪色,但是可以通过将平板放回碘显影室来使其重新显色.
高锰酸钾 - KMnO4
Detects molecules with an “oxidizable” functional group. Relatively insensitive, every thing stains yellow, frequently even without heating. Eventually, the entire plate will turn yellow and the spots will be indistinguishable from the background.
可以检测到具有“可氧化”官能团的分子。检测能力相对不敏感,化合物都会被染成黄色,甚至经常是在没有加热的条件下。最终,整个板都会的变成黄色,斑点和背景无法区分。
KMnO4(高锰酸钾)6 g
K2CO3(二氧化碳)40 g
5% aqueous NaOH(5%氢氧化钠水溶液)10 mL
H2O 600 mL
茚三酮染色剂 - Ninhydrin Stain
Especially sensitive to amino acids, as well as amines and anilines. Avoid contact with skin, or all of your friends will know that you have had an affair with nihydrin.
对氨基酸、胺和苯胺检测相当敏锐。避免进行皮肤接触,否则你所有的朋友都会知道你与尼三嗪有染。
0.25% of ninhydrin in water
0.25%的茚三酮
磷钼酸(PMA) - Phosphomolybdic Acid (PMA)
Everything stains blue–green. A very sensitive stain, possibly used most often. A 20% PMA in ethanol is commercially available; this should be diluted to 5% with ethanol.
会把化合物都染成蓝绿色。是一种非常灵敏的染色剂,也可能是最常用的染色剂。20%PMA的乙醇溶液可以在市面上买得到;在TLC实验中应该用乙醇将其稀释到5%。
Phosphomolybdic acid(磷钼酸) 12 g
Ethanol(乙醇) 500 mL
香兰素染色- Vanillin Stain
Different colors are manifested on heating for different molecules. Smells great, too (do not inhale intentionally!).
加热时不同的化合物表现不同的颜色。闻起来也很香(但不要故意吸入!)。
Vanillin(香草醛) 12 g
Ethanol(乙醇) 250 mL
Concentrated H2SO4(浓硫酸) 50 mL